How does the heart beat?
Every normal heartbeat begins with a specialized cluster of cells in the top right of the heart, called the SA node, generating an electrical impulse. This SA node is the pacemaker of the heart and generates this impulse 60-100 times per second under normal conditions. This impulse proceeds to spread down the heart’s electrical conduction system, first activating the upper chambers of the heart, then being slowed by the AV node, before activating the lower chambers of the heart (Park and Fishman, 2011).
Although we have just covered how electricity travels through the heart’s electrical conduction system in order to activate all the sections of the heart, we have not covered how the electrical impulse causes heart muscle to contract. The heart is composed of billions of heart muscle fibers called cardiac myocytes. The process of a cardiac myocyte being told to contract is called depolarization. Depolarization is a multi-step process that involves the cardiac muscle cell opening and closing channels and pumps to exchange electrolytes with the end goal of making the inside of the myocyte more negative than outside of the myocyte. In its relaxed state, the myocyte is more negative than the outside of the cell. Depolarization begins with sodium channels open and positive sodium ions rush in, causing the cell’s resting membrane potential to go from -70mV to +50mV.
The cell turning positive results in a sac in the myocyte called the sarcoplasmic reticulum to release large amounts of calcium into the cytoplasm, which causes the muscle to contract. In order to maintain the muscle contraction for the duration required, the cell closes its potassium channels and balances the cell voltage at +50 mV by striking a balance of letting positive calcium into the cell and letting positive potassium outside of the cell. After enough calcium gets into the cell, the calcium channels shut, calcium is pumped out of the cell, and potassium continues to leave the cell until it is negative again (Wei, 2023).
This is your Heart on Hypocalcemia
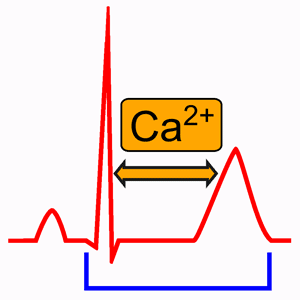
It should be clear that calcium is vital to the process of muscle contraction and repolarization. Calcium being released by the sarcoplasmic reticulum is the method by which muscle contracts (Nijjer and Dubrey, 2010). Because of this, a deficiency of calcium results in a worse cardiac contraction which causes reduced cardiac output. In addition, the influx of calcium into the cell is what eventually causes the cell to finish depolarization and start repolarizing. Because of this, hypocalcemia results in a reduced concentration gradient between the amount of calcium outside of the cell and the amount of calcium inside the cell, which makes calcium take longer to flow into the myocyte. Due to calcium taking longer to flow into the cell, it takes longer for the cell to reach the critical calcium intracellular concentration that causes the calcium channels to shut and repolarization to start. This is the mechanism by which hypocalcemia causes a prolonged QT interval. This prolonged QT interval results in an increased chance of R on T phenomenon which can precipitate dangerous arrhythmias like Torsades de Pointes (Grandi, 2009).
Interesting Aside on Calcium and the QT Interval
There is an interesting aside about the purpose of low calcium causing prolonged QT interval (and the opposite of high calcium causing a shortened QT interval). It is actually the mechanism by which the heart causes a shorter QT interval when it’s tachycardic and needs to start repolarizing faster before the next heartbeat and a longer QT interval when it’s bradycardic and has plenty of time to start repolarizing before the next heartbeat. Calcium levels build up around the extracellular area around the heart when it is tachycardic, causing localized hypercalcemia and a shortened QT interval, while calcium levels do not get the chance to build up around the heart when it is tachycardic, causing localized hypocalcemia and a prolonged QT interval (Yang, 1999).
Calcium, why so low?
All of this talk about what hypocalcemia does begs the question: how does hypocalcemia come to be in the first place? There are various causes including kidney problems which cause calcium to be lost in urine, loop diuretics (eg. Lasix) which also causes excessive excretion of calcium in urine, and nutritional deficiencies. That said, the largest cause of hypocalcemia is a malfunctioning parathyroid gland that is not producing enough parathyroid hormone or PTH (Stenman 2023).
The lesser known sister of the thyroid (but just as important!) is the parathyroid, which consists of 4 pea-sized glands behind the thyroid in the neck. PTH helps calcium absorption and prevents calcium loss in kidneys. PTH along with vitamin D are needed to absorb calcium from the GI tract (Yu, 2023). PTH stimulates active reabsorption of calcium in the distal tubule and connecting segment in the kidneys, preventing calcium excretion into the urine (Sterns, 2025).
Recognizing Hypocalcemia in the Field
Due to very few EMS agencies being able to run an i-STAT to directly measure serum calcium, it is difficult to confirm suspicion of hypocalcemia prehospitally. Simple reasons to suspect hypocalcemia include a prolonged QT interval, signs of cardiogenic shock with an unknown cause, and history of hypoparathyroidism. That said, there are specialized diagnostic tests for hypocalcemia called Chvostek’s and Trousseau’s signs. Chvostek’s sign involves tapping the facial nerve (located at the spot directly anterior to the earlobe) and checking to see if it induces a facial spasm.
The catch with Chvostek’s sign is that it is not very sensitive (only present in 2/3 of hypocalcemic patients) or specific (present in 10% of people with normal calcium levels). A better test is Trosseau’s sign, which is positive in 94% of patients with hypocalcemia and only in 1% of normocalcemic patients. Pay attention to your patient’s hand the next time the BP cuff inflates on their arm. If the ischemia induced by the cuff causes their hand to spasm, they are positive for Trousseau’s sign and hypocalcemia should be suspected (Jesus, 2012).
Hypocalcemia in the Real World
Let’s examine a real case study of a 58 year old female who presented to the ER with exertional dyspnea. She had a total thyroidectomy (unknown how much of the parathyroids were spared during surgery) 2 years ago to treat papillary thyroid carcinoma. Her blood work showed decreased PTH and low calcium. Her 12-lead showed further evidence of the effects of her hypocalcemia with a prolonged QTc interval. A cardiac ultrasound found an enlarged heart with a decreased left ventricular ejection fraction. If the cardiac ultrasound wasn’t enough evidence of how hypocalcemia was making her heart less effective, her BNP (a hormone released when the heart is stressed) was 12 times over the normal range. Unfortunately the doctors did not piece together that the low PTH was causing hypocalcemia, which was resulting in reduced cardiac output, causing her exertional dyspnea.
The doctors mistakenly thought congestive heart failure was the cause of her reduced heart function and symptoms and prescribed her a loop diuretic to treat the suspected CHF. The tragedy of her misdiagnosis doesn’t end with the fact that she didn’t get her low PTH and hypocalcemia treated, but the fact that loop diuretics cause increased calcium loss in the kidneys means her original treatment made her condition worse.
Luckily, on her second go around at the ER, the docs correctly identified the root cause of hypocalcemia secondary to reduced PTH production. The doctors immediately fixed her hypocalcemia with calcium gluconate, vitamin D, and the diuretic HCTZ. Calcium gluconate directly raises calcium levels and vitamin D helps the absorption of calcium in the GI tract. Now you may be wondering why the diuretic HCTZ was given if we just discussed how the last diuretic was worsening her calcium loss. This is because HCTZ is a thiazide-diuretic, which does not cause calcium loss like loop diuretics. If anything, HCTZ will cause increased serum calcium concentrations due to fluid loss in urine without calcium loss.
After treatment, her calcium returned to normal limits (and her QTc returned to normal as a result), echocardiography was normal again, and her ejection fraction improved. She continues to take calcium and vitamin D supplementation and her thiazide-diuretic and is living a healthy life. This woman’s story underlines the importance of knowing the physiology of hypocalcemia and the heart. If the second set of docs didn’t know about calcium and the heart, she could still be suffering to this day (Wen and Luo, 2022).
References
Fong, J., & Khan, A. (2012, February). Hypocalcemia: Updates in diagnosis and management for primary care. Canadian family physician Medecin de famille canadien. https://pmc.ncbi.nlm.nih.gov/articles/PMC3279267/
Grandi, E. (2009, March). Theoretical investigation of action potential duration dependence on extracellular Ca2+ in human cardiomyocytes. Science Direct. https://www.researchgate.net/publication/354209053_httpswwwsciencedirectcomsciencearticleabspiiS0304389421019920
Jesus, J. (2012, September 13). Chvostek’s and Trousseau’s signs | New England Journal of Medicine. New England Journal of Medicine. https://www.nejm.org/doi/full/10.1056/NEJMicm1110569
Nijjer, S., Ghosh, A. K., & Dubrey, S. W. (2010). Hypocalcaemia, long QT interval and atrial arrhythmias. BMJ case reports. https://pmc.ncbi.nlm.nih.gov/articles/PMC3029862/
Stenman, A. (2023). Hypocalcemia and its impact on Cardiovascular Health. https://www.hilarispublisher.com/open-access/hypocalcemia-and-its-impact-on-cardiovascular-health.pdf
Sterns, R. H. (2025, June 11). Diuretics and Calcium Balance. UpToDate. https://www.uptodate.com/contents/diuretics-and-calcium-balance
Wei, X. (2023, April 17). Physiology, cardiac repolarization dispersion and reserve. StatPearls [Internet]. https://www.ncbi.nlm.nih.gov/books/NBK537194/
Wen, Y., & Luo, X. (2022, September 12). Hypocalcemic cardiomyopathy: A case report. Frontiers. https://www.frontiersin.org/journals/cardiovascular-medicine/articles/10.3389/fcvm.2022.999550/full
Yang, B. (1999). Transmembrane ICa contributes to rate-dependent changes of action potentials in human ventricular myocytes. Am J Physiol Heart Circ Physio. https://journals.physiology.org/journal/ajpheart
Yu, E. (2023, August 14). Physiology, calcium. StatPearls [Internet]. https://www.ncbi.nlm.nih.gov/books/NBK482128/
Leave a Reply